Netra Sterilisation
Netra Sterilisation Introduction
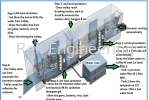
The multi-purpose trollies used by post-consumer for stacking various goods and baggage is which predominantly contaminated with hazardous bacteria’s (microorganism) which could be potential harmful for next customers. We at REK Engineering Machinery shall capitalize on the opportunities in sterilizing. These “trollies” and certified patented design with intellectual properties certification for sterilization system machine “NETRA™”. The Company shall act as a manufacturer for NETRA™.
REK Engineering Machinery Sdn. Bhd. is a company dedicated to support sanitation and hygiene in order to contribute in preserving nature or at the very least minimize the harms that we inflict on it in our everyday living.
Hygiene defined as a set of practices performed for the preservation of health. Hygiene is an old concept related to medicine, however today modern world it’s a one of the factors that we believe we can contribute is into commercially viable products, utilizing environmentally friendly hygiene trollies with an efficient systematic appliance as well as to personal and professional care practices related to most aspects of living. Regular hygienic practices may too considered good habits by a society while the neglect of hygiene to be considered disgusting, disrespectful or even threatening.
The trolleys are containers mainly used in large stores, grocery stores, supermarkets and airports to give customers and passengers the transport of selected goods and luggage without difficulty. Due to frequent use by the customers, these trolleys or shopping carts become dirty within a short time, so it is necessary for health and hygiene reasons that the used trolleys are must cleaned and washed at regular intervals.
Conventional methods currently apply by the shopping malls to wash trolleys or any other general-purpose had to be manually scrub and washed with the help of chemical cleaning agents or detergents.
Netra Sterilisation refers to the process of sterilizing eye surgical instruments and equipment. The term ''Netra'' refers to the Sanskrit word for ''eye.'' Sterilization is necessary to prevent the transmission of infectious agents such as bacteria, viruses, and fungi that can cause serious eye infections or even blindness.
The process of Netra sterilization typically involves several steps, including:
-
Cleaning: The instruments and equipment are first thoroughly cleaned to remove any debris, organic matter, or contaminants that may be present on their surfaces. This can be done manually or with the help of automated cleaning equipment.
-
Disinfection: After cleaning, the instruments are disinfected using a high-level disinfectant solution that is specifically designed for use on medical devices. The disinfectant solution is usually left in contact with the instruments for a specified period of time to ensure complete sterilization.
-
Sterilization: Once the instruments have been disinfected, they are typically sterilized using a method such as autoclaving, which involves exposing them to high temperatures and pressure to kill any remaining microorganisms. Other sterilization methods such as chemical sterilization or dry heat sterilization may also be used depending on the type of instruments and equipment being sterilized.
-
Packaging: After sterilization, the instruments and equipment are typically packaged in sterile packaging material to maintain their sterility until they are ready for use in surgical procedures.
Netra sterilization is a critical component of eye surgery and is essential for ensuring patient safety and preventing the spread of infections. Sterilization processes must be carefully monitored and validated to ensure that they are effective in eliminating all harmful microorganisms.